HCMC launches clinical trial to treat brain injury using vagus nerve stimulation
“Electroceutical” Treatment Hopes to Stimulate Brain Healing Without Medications
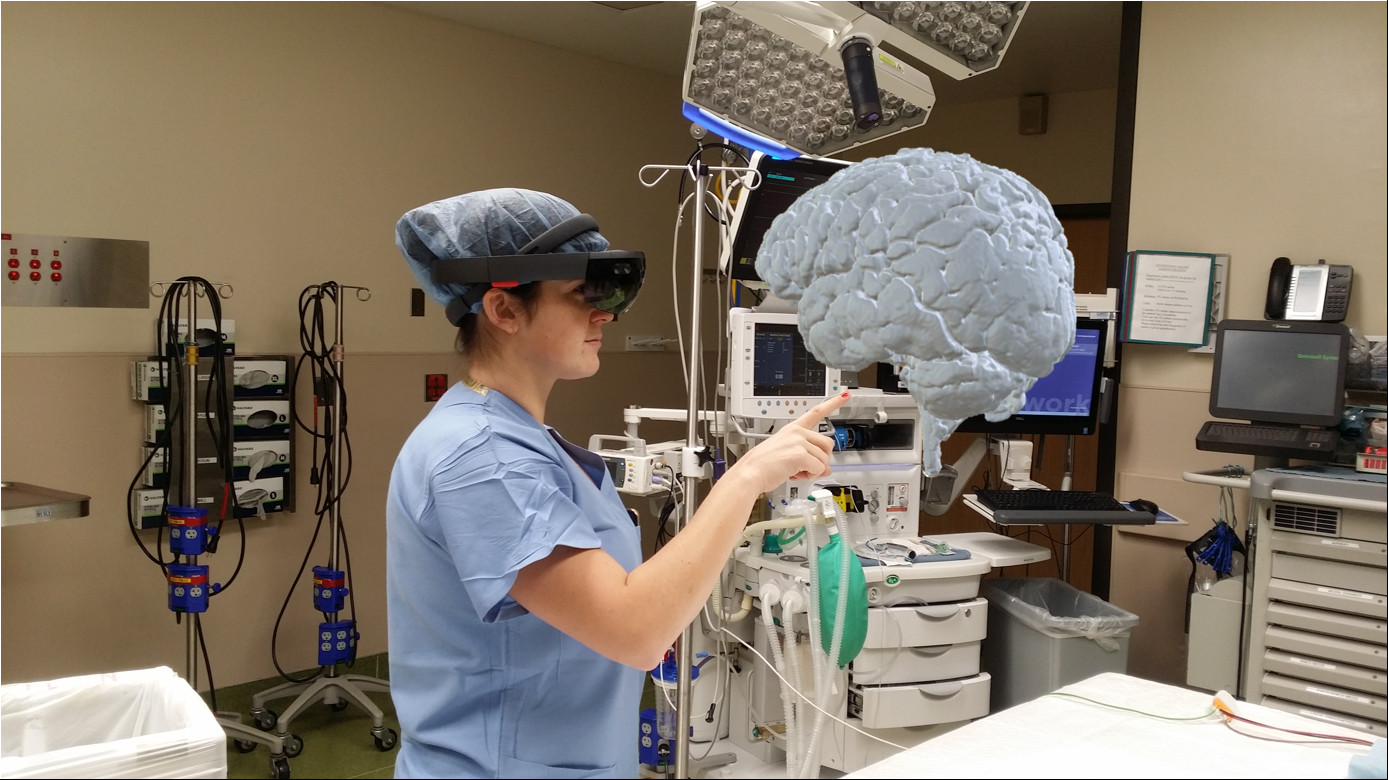
Dr. Molly Hubbard, lead investigator of the VANISH TBI study examines a 3D hologram of the injured patient’s brain. The image was generated by Dr. Abdullah Bin Zahid in the HCMC Brain Injury Research Laboratory.
A simple slip on the ice while crossing a parking lot in downtown Minneapolis triggered the problem for 47-year-old John Doe (not his real name). By the time emergency medical personnel arrived he knew his own name, but did not remember falling, the date, or even where he was. “I didn’t even know what had happened until I had been in the hospital for three days,” he said.
Brought into Hennepin County Medical Center (HCMC), John had classic brain injury, with tiny amounts of blood on the surface of his brain and interspersed into its folds. Radiology studies were consistent with brain trauma, but the injury was not severe enough to require surgery. Several days after the accident he moved into the brain injury rehabilitation unit at HCMC.
“I’m still trying to figure out how to get back to my normal life. It’s … hard to believe that this is my life. A lot of symptoms are starting to get better but they’re still there. I have problems remembering words,” said John, who works as a professional writer.
Luckily for John, HCMC, in conjunction with the University of Minnesota, has just launched a new clinical trial to treat brain injury, and he was the second patient to be enrolled. He remarked, “I really like the idea of being able to help others with brain injury; maybe even help people who have it worse off than me.”
Traumatic brain injury is the most common cause of death and disability in Americans under the age of 35 years and is most typically caused by car accidents and falls. Increased recognition of the consequences of brain injury has arisen from several large epidemiologic studies. A Swedish study of more than one million subjects, of whom approximately 10% had a brain injury in childhood or adolescence, showed an increased likelihood for psychiatric disorder, disability pension requirement and premature death in people who had a brain injury compared to their siblings.1 A Canadian epidemiologic study showed an association between concussions and increased risk of suicide; where the risk of suicide was higher in subjects with multiple concussions. 2
Despite the known significant consequences of brain injury, few effective treatments have been validated. This problem may be attributed, at least in part, to the fact that objective measures and a classification scheme characterizing the complex nature of brain injury still need to be developed. The HCMC Brain Injury Research Lab, headed by University of Minnesota Associate Professor and neurosurgeon Dr. Uzma Samadani and Chief of Neurosurgery Dr. Thomas Bergman, recently launched the nation’s largest single center study to develop a classification scheme for brain injury using objective measures such as eye tracking, serum markers and MRI to characterize the injuries. The study, called CLASSIFY TBI, is funded by global healthcare company Abbott (Abbott Park, Illinois) along with Minnesota state legislature funding, and the Rockswold Kaplan Endowment for Traumatic Brain Injury Research. It enrolled more than 250 patients and control subjects in its first six months.
As a follow-on to that study, HCMC intends to start several clinical trials in 2017 for treatment of specific subtypes of brain injury, focusing on modalities with compelling and efficacious mechanisms, extensive pre-clinical and/or clinical data, and the capability to be widely disseminated within a relatively short time. Enrollment in these trials will be limited to patients with objectively measured brain injury.
Vagal Activation of NeuroImmune Systems to Heal Traumatic Brain Injury (VANISH TBI) is the first of these trials to be launched. The underlying premise of the treatment is that stimulation of the vagus nerve, which runs along the side of the neck and has projections to multiple areas in the brain cortex, can reduce the consequences of brain injury. Vagus nerve stimulation is known to reduce seizures in patients with epilepsy and improve symptoms of depression in patients with pharmacologically refractory disease.
“Most patients with brain injury do not require surgery,” said Dr. Sarah Rockswold, medical director of the Traumatic Brain Injury Center at HCMC and a co-investigator on both the CLASSIFY TBI and VANISH TBI studies, “But that does not mean that their problems are less significant than those with surgical needs.”
“We hope that nerve stimulation will help restore failed circuitry resulting from specific types of injury,” said Dr. Samadani. “When I originally wrote a grant to the Department of Defense to propose treatment of severely brain injured subjects with vagus nerve stimulation in 2006, the only stimulators available for such treatment had to be surgically implanted in the neck with a battery pack put into the chest wall.”
The study was finally funded to take place in New York by the Department of Veterans Affairs in 2010 but was terminated after Hurricane Sandy closed the Manhattan VA for seven months in 2012. Dr. Samadani moved to HCMC and the University in 2015 and resubmitted for FDA Investigational Device Exemption approval and Minnesota state funding, in conjunction with lead study investigator and neurosurgery resident Dr. Molly Hubbard.
“By then a new device for vagus nerve stimulation became available – one that stimulates the nerve transcutaneously through the skin, thus not requiring surgical implantation. The use of a non-invasive device carries less risk,” said Dr. Samadani, “and thus we targeted the study to a different and broader subgroup of patients with brain injury rather than restricting it to the most severe and refractory cases.” The device called gammaCore® is CE cleared in Europe for treatment of cluster headache and migraine and manufactured by the New Jersey based company Electrocore LLC.
The VANISH TBI pilot study will enroll a total of 30 patients to undergo twice daily two-minute treatments with the gammaCore® device, or a sham for comparison. Patients and care providers will be blinded to who is receiving treatment stimulation versus sham. Participants will be followed for 12 weeks for serial assessment both symptomatically and using objective measures. The study will examine whether the use of the gammaCore® device can improve recovery from moderate TBI and decrease brain injury symptoms such as headaches, memory loss, slow thinking (impaired cognition), seizures, inability to sleep, and depression. If the study shows efficacy, a larger multicenter trial will be proposed.
“Eleven years from grant proposal to first patient treated is a long time,” said Dr. Bergman, “but the interval development of potentially better stimulation technology, as well as improved capability for classification of injury and outcome assessment should hopefully increase the likelihood of trial success. We will soon find out if this treatment was worth the wait.”
“Electroceuticals are currently a very new and exciting treatment modality,” added Dr. Hubbard. “Concerns about unwanted side effects and addiction with conventional medications have led both pharmaceutical companies and the National Institutes of Health to invest very heavily in various modalities for central and peripheral nervous system stimulation.” Dr. Hubbard recently penned an article in AANS Neurosurgeon about increased use of electroceuticals to treat neurologic disease (http://aansneurosurgeon.org/features/impact-injury-five-trends-watch/). “From the military to sports, brain and spine trauma, electroceuticals are being used to investigate different ways to modulate the nervous system and return normal function.”
“Neuromodulation is a nascent field with the potential to treat many neurological disorders where pharmaceuticals fall short,” says Dr. Matthew Britton, a neurology resident involved in interpreting EEG findings from the study. In addition to the brain injury team at HCMC, specialized neurologists (epileptologists) from the University of Minnesota are interpreting EEG findings from before and after the treatment periods in an attempt to quantify changes seen on EEG after the treatment period. “We are building on an existing strong collaboration between the neurology and neurosurgery departments both at HCMC and at the University of Minnesota,” said Dr. Britton. “This represents what will most likely be one of the most important topics in neuroscience over the next several decades.”
The VANISH TBI clinical trial is funded by the Minnesota state legislature’s Spinal Cord Injury and Traumatic Brain Injury research grant program, and HCMC Rockswold Kaplan Endowment for TBI Research. The gammaCore® devices are provided by Electrocore LLC. Dr. Samadani, HCMC, New York University, and the Department of Veterans Affairs all have a proprietary interest in the investigational eye tracking technology, licensed by Oculogica Inc, and being used for injury classification and outcome assessment.
1 Sariaslan, A., Sharp, D. J., D’Onofrio, B. M., Larsson, H. & Fazel, S. Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLOS Medicine 13, e1002103, doi:10.1371/journal.pmed.1002103 (2016).
2 Fralick, M., Thiruchelvam, D., Tien, H. C. & Redelmeier, D. A. Risk of suicide after a concussion. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 188, 497-504, doi:10.1503/cmaj.150790 (2016).
About Hennepin County Medical Center
Hennepin County Medical Center (HCMC) is a 484-bed comprehensive academic medical center and public teaching hospital located in downtown Minneapolis. It was one of the first hospitals in the country to be verified as a Level I Trauma Center and continues to be a leader in providing trauma care – assessing more than 22,000 trauma patients each year. As Minnesota’s first Level I Adult and Pediatric Trauma Center, HCMC admits and treats more traumatic brain injuries than any other hospital in Minnesota. To learn more, visit www.hcmc.org/braininjury.
About the University of Minnesota Medical School
The University of Minnesota Medical School is at the forefront of learning and discovery, transforming medical care and educating the next generation of physicians. Our graduates and faculty produce high-impact biomedical research and advance the practice of medicine. Learn how the University of Minnesota is innovating all aspects of medicine by visiting www.med.umn.edu.

